Full Story: BEST Magazine (UK) (7/16), AZoM (7/8)
New membrane to extract lithium from water

Researchers at the US Department of Energy’s Argonne Laboratory have developed a membrane technology that extracts lithium from water.
The researchers say that most of the Earth’s lithium is dissolved in seawater and underground saltwater reserves as opposed to the hard-rock and salt lakes where most lithium is mined from. But extracting it has been expensive and inefficient.
A membrane was made to filter out other elements from salt water, which exist as cations that have lost one or more electrons. The other cations are filtered out based on both size and degree of charge.
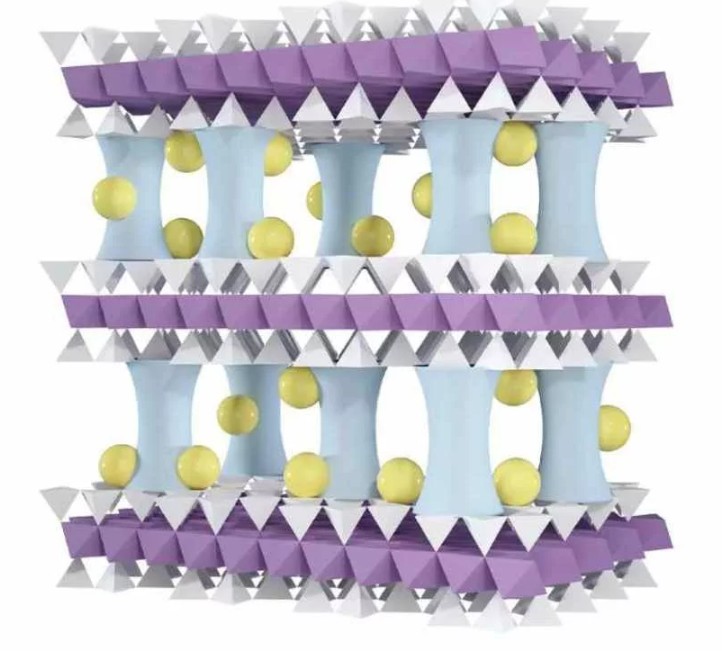
It is made from a naturally abundant clay called vermiculite that costs around $350/ton.
The researchers developed a process to peel apart the clay into thin layers, which are a billionth of a metre thick, and restack them to form a filter.
To prevent the clay layers from falling apart in water, the researchers added microscopic aluminium oxide pillars between the layers to hold them in place, to neutralise the membrane’s negative surface charge.
Sodium cations were introduced to capture lithium ions in the water more easily whilst keeping out magnesium ions. The surface charge would change from neutral to positive.
For further refinement, more sodium ions were added to decrease the membrane’s pore size. It allows for smaller ions like sodium and potassium to pass through whilst catching larger lithium ions.
“Filtering by both ion size and charge, our membrane can pull lithium out of water with much greater efficiency,” said first author Yining Liu, a Ph.D. candidate at UChicago and a member of the AMEWS team. “Such a membrane could reduce our dependence on foreign suppliers and open the door to new lithium reserves in places we never considered.”
Image: An illustration of the vermiculite membrane with aluminium oxide pillars and doped sodium ion. Credit: Argonne Laboratory.